Weight Loss Injections
Leading Exporters, Wholesaler and Trader of 10mg Mounjaro Tirzepatide Injection, 15mg Mounjaro Tirzepatide Injection, 6mg Saxenda Liraglutide Injection, Igtropin Injection, Mounjaro 10mg Injection, Mounjaro 12.5mg Injection, Mounjaro 15mg Injection, Mounjaro 2.5mg, Mounjaro 2.5mg Injection, Mounjaro 5mg Injection, Mounjaro 7.5mg Injection, naltrexone bupropion hcl tablets, Ozempic Injection, Saxenda 6mg Injection, Wegovy 0.25mg Injection, Wegovy 0.5mg Injection, Wegovy 1mg Injection and Zepbound Injection (5 Mg / 7.5 Mg / 10 Mg) from George Street.
| Business Type | Exporter, Supplier, Retailer, Distributor, Importer |
| Size | Compact, handheld pre-filled injection pen |
| Type | Pre-filled single-use injector pen |
| Material | Pen body: Medical-grade plastic |
| Color | Pen casing is typically off-white or light gray |
| Quantity | Usually sold in a box containing 1 or 4 pre-filled pens, depending on pharmacy and country Each pen |
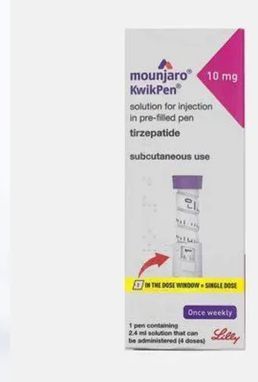
| Strength | 10 mg of tirzepatide per 0.5 mL injection Part of the standardized dosing range (2.5 mg → 15 mg) |
| Flavor | Injectable medications do not have flavors. Mounjaro is a clear, colorless liquid designed for inje |
| Warranty | Manufacturer quality guarantees Expiration date Temperature-storage guidelines Replacement polici |
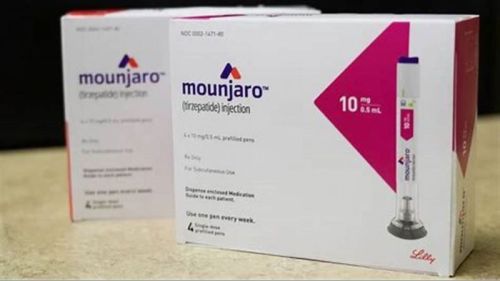
Mounjaro (tirzepatide) is a prescription-only medication supplied as a once-weekly injectable solution. It contains tirzepatide, a novel dual GIP and GLP-1 receptor agonist, designed to support improved metabolic control when used under the guidance of a licensed healthcare provider.
The 10 mg strength is one of the mid-range dose levels in the Mounjaro pen lineup and is typically used only after a person has safely progressed through the lower strengths.
| Business Type | Exporter, Supplier, Retailer, Distributor, Importer |
| Feature | As A Result, Mounjaro Lowers Both Fasting And Post‑meal (postprandial) Glucose Levels. Guideline Ce |
| Purity | Excipients / Non‑active Ingredients (in The 0.5 ML Solution) Include: Sodium Chloride, Sodium Phosph |
| Usage | Administration: Once Weekly, By Subcutaneous Injection (under The Skin), In The Abdomen, Thigh, Or U |
| Dosage Form | Sterile Solution For Subcutaneous Injection, Pre-filled Pen Or Single-dose Vial |
| Form | Liquid Solution Pre-filled Injection Pen |
| Color | Clear, Colorless Solution In Pre-filled Pen Pen Casing: Typically Light Gray Or White With Labeled |
| Brand Name | Mounjaro® |
| Origin | Manufactured In USA / Eli Lilly Facilities Under CGMP Standards |
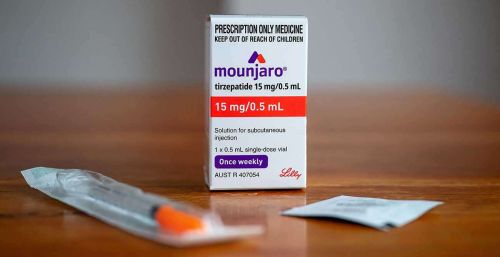
Mounjaro® is a prescription medication that contains tirzepatide, a dual GIP/GLP-1 receptor agonist. It is approved for the management of type 2 diabetes mellitus in adults. It improves blood sugar control by enhancing insulin secretion, reducing glucagon, and slowing gastric emptying.
| Business Type | Exporter, Supplier, Retailer, Distributor, Importer |
| Type | Injection |
| Purity | Pharmaceutical-grade purity |
| Age Group | Adults (18 years and above) |
| Quantity | 1 pre-filled pen per pack |
| Strength | 6 mg per pen |
| Form | Pre-filled injection pen |
| Composition | Liraglutide (recombinant GLP-1 analog) |
| Active Ingredient | Liraglutide |
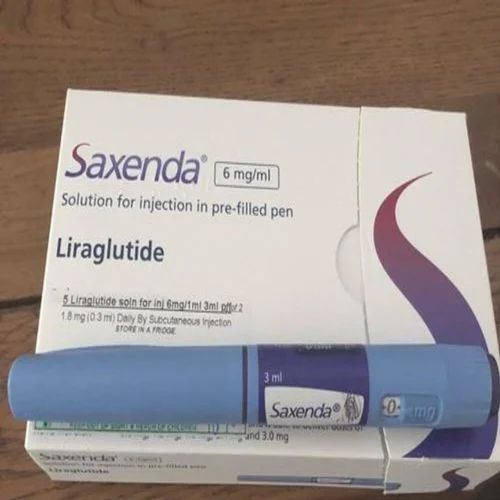
It is formulated as a pre-filled injection pen for subcutaneous administration and is primarily used as a prescription medicine for weight management in adults with obesity or overweight conditions under medical supervision
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
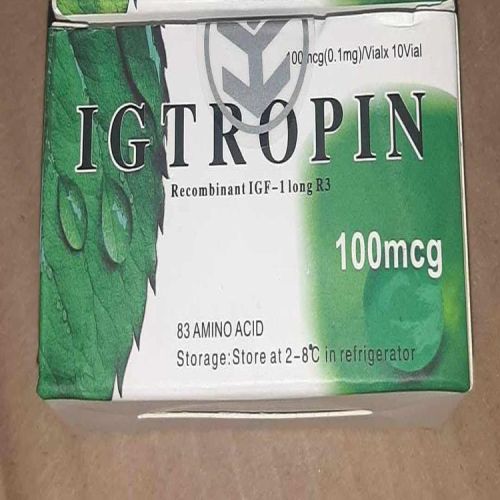
| Strength | 100 mcg |
| Composition | Amino Acid |
| Storage | Cool & Dry Place |
| Application | Muscle Regeneration |
| Packaging Size | 10 Vials |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Strength | 10 mg |
| Composition | Tirzepatide |
| Storage | Cool & Dry Place |
| Application | Obesity Control |
| Packaging Type | Prefilled |
| Manufacturered By | Eli Lilly |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
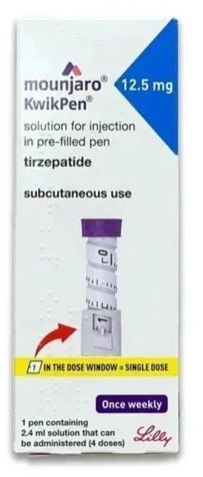
| Strength | 12.5 mg |
| Composition | Tirzepatide |
| Storage | Cool & Dry Place |
| Application | Advanced Weight Loss |
| Packaging Type | Prefilled |
| Manufacturer | Eli Lilly |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Strength | 12 mg |
| Composition | Tirzepatide |
| Storage | Cool & Dry Place |
| Application | Dose Therapy |
| Packaging Type | Prefilled |
| Manufacturer | Eli Lilly |
| Business Type | Exporter, Supplier, Retailer, Trader, Distributor, Importer |
| Form | Injectable solution (pre-filled pen) |
| Packaging Type | Box containing single-dose prefilled injection pens |
| Brand Name | Mounjaro® |
| Usage/Application | Hospital |
| Model Number | Mounjaro 2.5 mg/0.5 mL Prefilled Pen |
| Country of Origin | Made In India |
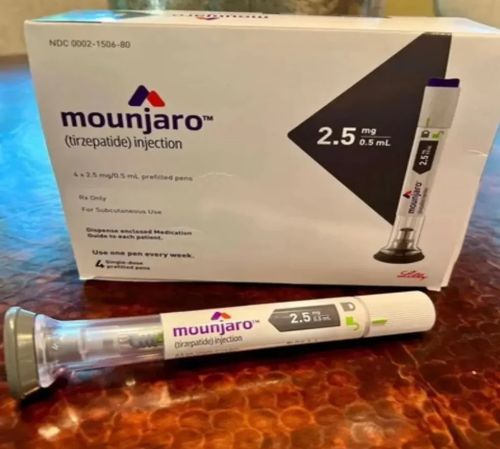
Mounjaro 2.5 mg refers to the 2.5 milligram starting dose of Mounjaro, a prescription injectable medicine whose active ingredient is tirzepatide. It’s given as a once-weekly subcutaneous injection (under the skin, often in the abdomen, thigh, or upper arm) and is primarily prescribed to improve blood sugar control in adults with type 2 diabetes. When used with diet and exercise, it can also support weight loss.
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Type | Allopathic |
| Strength | 2.5mg |
| Composition | Tirzepatide |
| Storage | Cool & Dry Place |
| Application | GLP1 GIP Dual Agonist |
| Packaging Type | Prefilled |
| Manufacturered By | Eli Lilly |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Type | Allopathic |
| Strength | 5 mg |
| Composition | Tirzepatide |
| Storage | Cool & Dry Place |
| Application | Appetite Regulation |
| Packaging Type | Prefilled |
| Manufacturer | Eli Lilly |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Type | Allopathic |
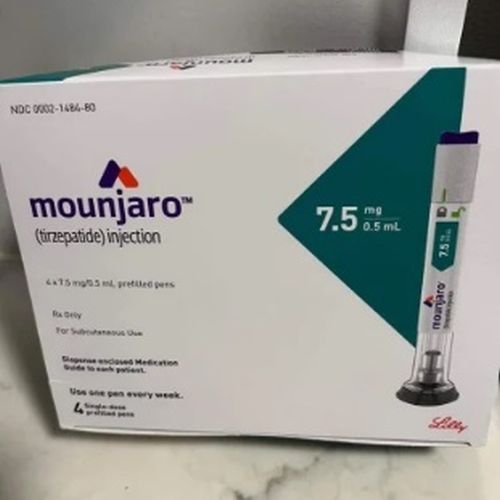
| Strength | 7.5 mg |
| Composition | Tirzepatide |
| Storage | Cool & Dry Place |
| Application | Weight Reduction |
| Packaging Type | Prefilled |
| Manufacturer | Eli Lilly |
| Business Type | Exporter, Supplier, Retailer, Distributor, Importer |
| Brand Name | Contrave® |
| Grade Standard | Pharm Grade |
| Form | Tablets |
| Packaging Size | 8mg/90mg |
| Usage | Fat Loss |
| Color | Blue |
| Packaging Type | Plastic Container |
| Prescription | Prescription |
Naltrexone–bupropion, marketed under the brand name Contrave®, is a prescription oral medication approved for chronic weight management in adults. It is used as an adjunct to a reduced-calorie diet and increased physical activity.
| Business Type | Exporter, Supplier, Trader |
| Injection Type | Prefilled |
| Form | Liquid |
| Usage | Weight Control |
| Manufacturered By | Novo Nordisk |
| Generic Name | Ozempic |
| Composition | Semaglutide |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Strength | 6 mg |
| Composition | Liraglutide |
| Storage | Cool & Dry Place |
| Application | Obesity Management |
| Packaging Type | Prefilled |
| Manufacturered By | Novo Nordisk |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Medicine Type | Allopathic |
| Strength | 0.25mg |
| Composition | Semaglutide |
| Storage | Cool & Dry Place |
| Usage | Obesity Management |
| Packaging Type | Prefilled |
| Manufacturered By | Novo Nordisk |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Medicine Type | Allopathic |
| Strength | 0.5mg |
| Composition | Semaglutide |
| Storage | Cool & Dry Place |
| Usage | Appetite Control |
| Packaging Type | Prefilled |
| Manufacturered By | Novo Nordisk |
| Business Type | Exporter, Supplier, Trader |
| Form | Liquid |
| Medicine Type | Allopathic |
| Strength | 1 mg |
| Composition | Semaglutide |
| Storage | Cool & Dry Place |
| Usage | Obesity Treatment |
| Packaging Type | Prefilled |
| Manufacturered By | Novo Nordisk |
| Business Type | Exporter, Supplier, Retailer, Trader, Distributor, Importer |
| Origin | Developed and manufactured by Eli Lilly and Company |
| Brand Name | Zepbound |
| Color | Clear, colorless solution |
| Form | Liquid solution |
| Dosage Form | Available strengths include 5 mg, 7.5 mg, and 10 mg |
| Usage | Indicated for chronic weight management in adults with obesity or overweight with related conditions |
| Purity | Manufactured under strict quality controls with high peptide purity and sterility assurance |
| Feature | Dual GIP and GLP-1 receptor agonist (tirzepatide) |
Zepbound is a prescription injectable medication containing tirzepatide, a dual GIP and GLP-1 receptor agonist. It is supplied as a single-dose, pre-filled injection pen and is approved for chronic weight management in adults with obesity or overweight with related medical conditions, under medical supervision.
Zepbound® is available in multiple strengths, including 5 mg, 7.5 mg, and 10 mg, allowing for gradual dose adjustment as prescribed. The injection is formulated as a clear, colorless solution intended for subcutaneous administration.
Manufactured by Eli Lilly and Company, Zepbound® is a prescription-only medicine, regulated and distributed according to regional health authority approvals.